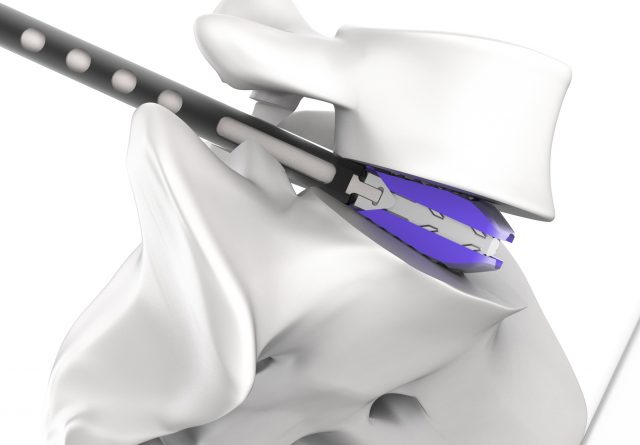
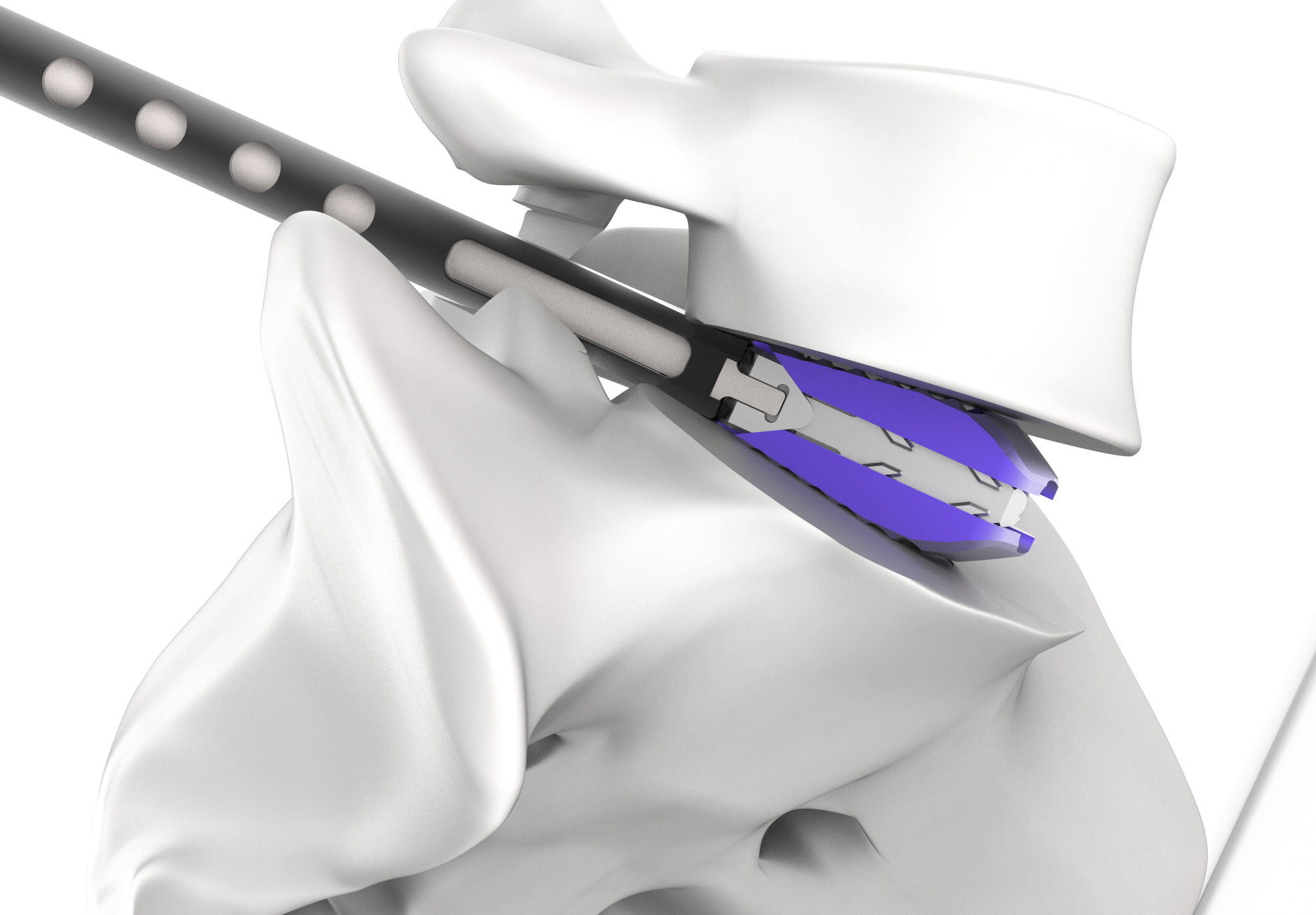
On January 18, bio-regenerative medicine company CGBIO (CEO Hyun-seung Yu) announced that the company had launched the next-gen expandable cage ‘Excender’.
Spinal fusion is a surgery to stabilize the spine where discs damaged by internal and external causes are removed and the alternative bone substitutes are filled inside the cage, a structure inserted to substitute the disc(s) removed for appropriate spine height and angle, and the upper and lower spines are fused into a single bone structure.
But to insert a cage in the space between spine discs during actual surgeries, the cage must pass through a very narrow gap between just 2~5mm in height while the cage must be 9~14mm in height to restore the original height of the replaced disc.
But for the existing cages that were large, it was difficult to put them through narrow spaces, which resulted in the potential risk of damaging the spine during the insertion process. For the existing cages that were small, it was difficult to restore the original height and angle of the replaced disc. This resulted in the need for the development of a next-gen expandable cage that can expand sufficiently inside the body after being inserted initially in a small form factor.
Excender was developed in a pellet form in the front to conveniently access the narrow space. The product is available in two lengths (28mm and 32mm) and it can be used selectively depending on the spine area and can expand up to 4mm after being inserted at the initial height of 8mm. Through such process, Excender offers the advantage of minimizing the risk of damaging the spine during cage insertion and sufficient expansion to restore the original height and angle of the disc.
The core competitiveness of Excender is in its ‘alternative bone substitute injection function’ and ‘shielded structure’.
For other height expanding cages in the market, it was difficult to fill up the alternative bone substitute in the empty space generated after its expansion. Also, they were manufactured in an all-around open form, which raised concerns about a potential decline in bone fusion efficacy for the alternative bone substitute.
Bue Excender offers height expandability along with the function to allow the injection of alternative bone substitutes in the empty space generated after expansion and a shielded structure. This allows the surgeons to fill up the empty space caused by missing discs with a significant amount of alternative bone substitute materials and minimizes the risk of losing the alternative bone substitute after surgery. Therefore, patients can expect a more positive outcome after the surgery for spinal fusion, which is the ultimate goal of spinal fusion surgery.
Also, Excender has maximized the area making contact with the edge of the bone with the highest bone density among all vertebrae for stable support, which minimizes the risk of the cage collapsing from within the interior side of the bone that is relatively weaker compared to its exterior side. Excender was recognized for such technological innovation and it was patented for its expandable cage technology in October 2021.
It is also noteworthy that Excender was registered for the National Health Insurance benefits. Expandable cages from global medical appliance manufacturers are known to be prescribed at prices that are more than 5 times the price of the non-expandable cages in the overseas market due to their high difficulty in development and superb performance. But Excender became available as a benefit for the National Health Insurance policyholders in Korea in July 2022, which allows the patients to be prescribed Excender at a price similar to the non-expandable cage upon meeting the benefit requirements.
CEO Hyun-seung Yu stated, “Expandable cages employ complex technology and require high development and manufacturing costs, so it is true that other similar products overseas were unable to penetrate the domestic market in Korea, which is under the effect of relatively lower insurance fees. But if this continues, the damages will be suffered by the patients.” He continued, “Excender is an expandable cage with superior performance with other global companies overseas requesting us for distribution rights. We have launched this product in both overseas and domestic markets to offer optimal treatment options for domestic patients.”
He added, “The trend in spinal implants is shifting from non-expandable cages to expandable cages across the globe and this launch of Excender will be not only able to lead the era of expandable cage overseas but also be used with osteoplastic protein alternative bone substitute products from our company to provide treatment options that can offer superb surgery results for patients who require spinal fusion in the future.”