

EXCENDER
Expandable Lumbar interbody spacer / Bone graft injectable & Expandable TLIF cage
-
Overview
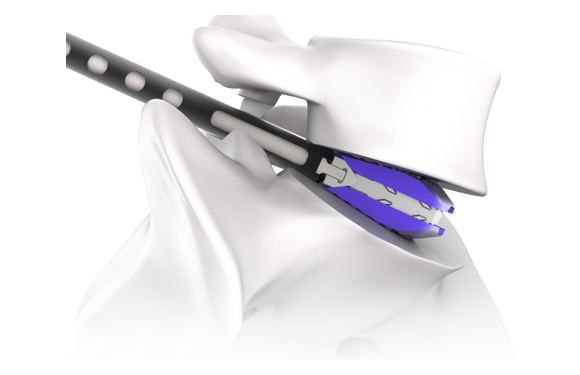
The EXCENDER is an interbody fusion cage made from a titanium alloy that complies with ASTM F136 standards. This product is specifically designed for use in Transforaminal Lumbar Interbody Fusion (TLIF) procedures to address spinal disorders, particularly in patients with damaged discs. The EXCENDER is intended for implantation in the lumbar region to facilitate spinal fusion, addressing structural abnormalities of the lumbar spine. It can be used in conjunction with various bone graft materials. The implant is initially inserted between the vertebrae in a collapsed state and can be expanded to the desired height using surgical instruments after insertion.
-
Intended Use
The EXCENDER is a cage-type device used for interbody fusion in the lumbar spine to treat structural abnormalities that require bone fusion. It is implanted in the intervertebral space of the lumbar spine and can be used alongside bone graft materials.
-
Pre-Use Preparation
- Inspect the packaging for any signs of damage and confirm that the product is within its expiration date to ensure sterility.
- After removing the packaging, check for any damage to the device.
- Healthcare professionals must be thoroughly familiar with the surgical techniques, indications, and contraindications associated with this product.
- The surgeon must have a comprehensive understanding of the surgical procedure using this product’s instruments, the clinical indications, contraindications, and potential complications. The surgeon should also inform the patient of these indications, contraindications, precautions, and potential adverse effects.
- Assess factors such as the patient’s age, medical history, and bone condition. Ensure that there are no biological or biomechanical factors that could compromise the surgical outcome.
- Carefully read and follow the instructions for use.
-
Operating Instructions
- Position the patient appropriately on the operating table and administer anesthesia.
- Make an incision at the surgical site and carefully dissect the soft tissues to access the surgical area.
- Use surgical instruments to remove the intervertebral disc and create space for the cage.
- Select the appropriate size and angle of the implant using trial and measuring tools.
- Insert the cage posteriorly into the prepared intervertebral space using an implant holder.
- Expand the implant to the desired height using an implant driver.
- To promote successful spinal fusion, fill the cage with autograft or bone graft material.
- Perform a final X-ray to confirm the correct placement of the cage.
- The product must not be reused under any circumstances. Even if the device appears undamaged after removal, pressure and tension during use may cause micro-defects or internal deformations, leading to potential product failure.
-
Post-Use Storage and Handling
- This product is a single-use medical device and must not be reused.
- Any unused product after opening must be properly disposed of.
