CGBio (CEO Hyun Seung Yu), specializing in bio regenerative medicine, announced on the 14 the acquisition of FDA 510k clearance (pre-market approval) for its Advanced LumFix Spinal Fixation System (hereinafter referred to as the “Advanced LumFix”).
The FDA clearance for Advanced LumFix came in about one year after CGBio submitted its application in July 2022. This is expected to speed up the company’s entry into the US spine implant market through CGMedTech, its American subsidiary incorporated in the first half of this year.
CGBio plans to focus on three major businesses in the US through CGMedTech: ▲bone and spine implants, ▲3d printing, and ▲human tissue business. Advanced LumFix is a part of bone and spine implants business, one of the three major businesses. The FDA clearance provides a blueprint for the company’s entry into the US spine implant market.
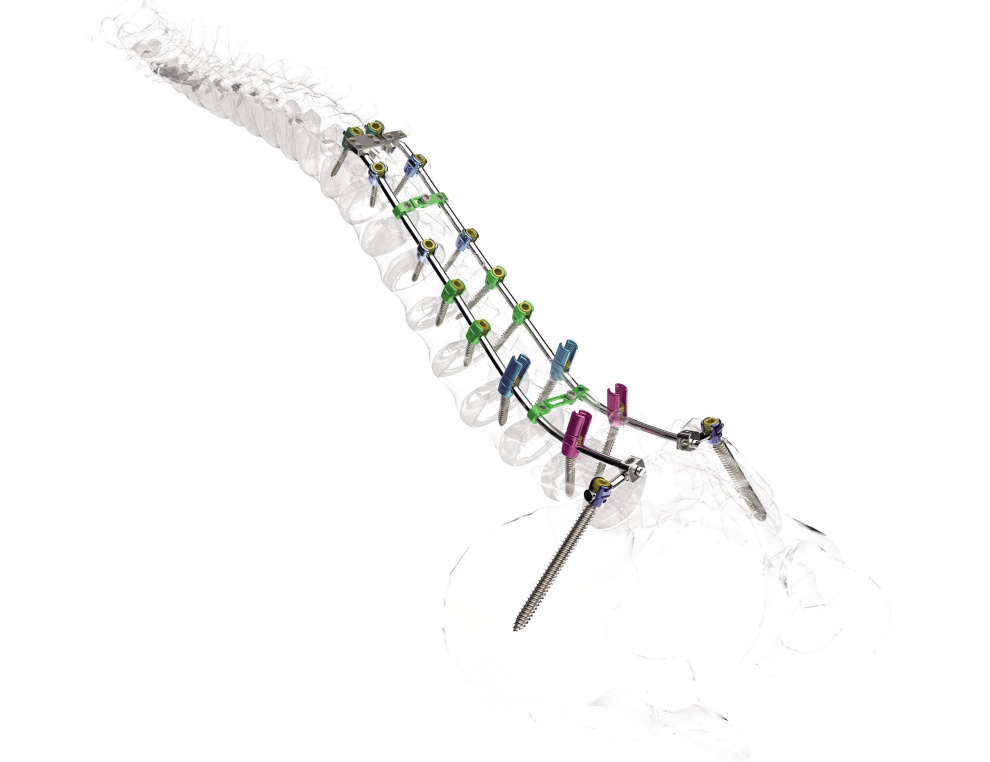
LumFix, released in the nation in 2013, is a device that fixes the spines of patients with spinal deformities to prevent further deformations and restore the original spinal angle. In 2021, it was rebranded as Advanced LumFix after the addition of a surgical tool that enhances the convenience of patients and medical specialists. Advanced LumFix is the only domestic spine implant that comes with a spinal corrective surgical tool. Since its release into the market, it has been widely used and recognized for its safety and efficacy.
Advanced LumFix is approved for use in spine fusion surgery for the purpose of correcting spinal deformities and treating spinal lesions. Spinal damages or deformities caused by degenerative diseases or accidents are typically treated with drug treatment and conservative treatment. However, if these treatments are not effective or spinal damage continues, spine fusion surgery is performed to connect the spinal bones above and below the affected area. It can also be used to correct the spines of scoliosis patients that are bent beyond the normal range.
In particular, Advanced LumFix comes with a hook that can be used in areas where it is hard to insert a screw or to support a screw that is not sufficiently fixed. The hook can be adjusted in angle, which allows it to be customized to different shapes of spines. Advanced LumFix is the nation’s only spinal corrective implant that comes with an adjustable-angle hook.
Additionally, it contains nine connectors that firmly hold between rods, and between rods and screws, which allows a firmer fixation between the components. Furthermore, it comes with a spinal angle corrective surgical tool called Multi Deformity Reducer (MDR). With its comprehensive set of materials and tools necessary for spine fusion surgery, Advanced LumFix is expected to increase the convenience of American medical specialists.
CGBio CEO Hyun Seung Yu said, “Advanced LumFix is a next-generation spinal corrective surgical solution that has gained significant validation in the domestic spine implant market. With the recent FDA clearance, we are now fully prepared to enter the US,” adding, “We hope that Advanced LumFix can be an innovative and effective solution for degenerative disc patients as the disease is increasing across the world, including the US.”
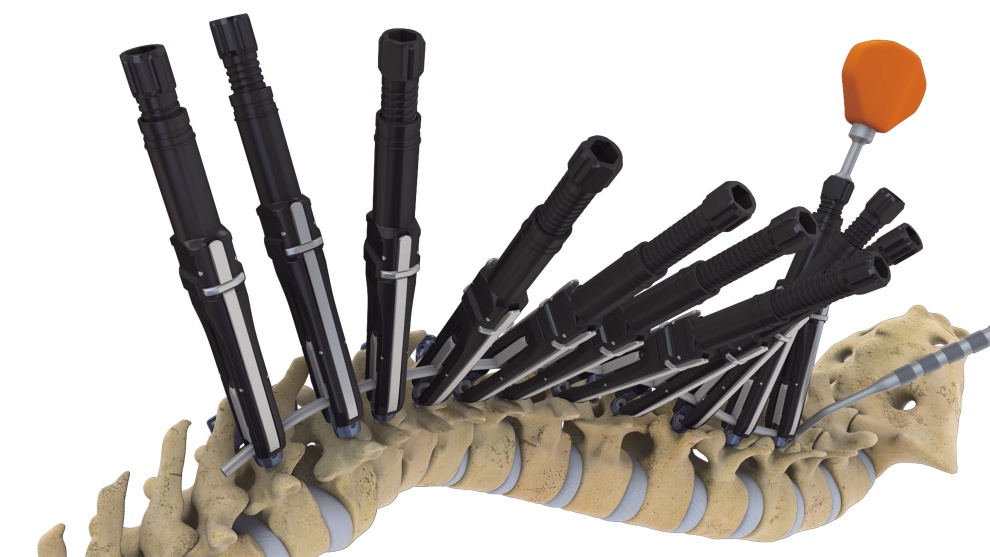
▲ LumFix’s MDR applied to correct the spinal angle