Bioceramics of CGBIO Hydroxyapatite
Hydroxyapatite (Ca10(PO4)6(OH)2, CAS No. 1306-06-5 NF 규격준수)는
시린이 전용 치약, 골대체제와 화장품 원료로 사용 중인
인체에 안전한 바이오 원료입니다.
-
Qualified as Medical Grade
Qualified standards of USP-NF(US Pharmacopeia-National Formulary) or ASTM 1185-03 for Medical Device & Medicine
-
Obtains Various Form
Has various HAP forms including Nano size(Able to control bead porosity)
-
Verified Safety
Sold as various product types in several countries for 15 years
SPEC
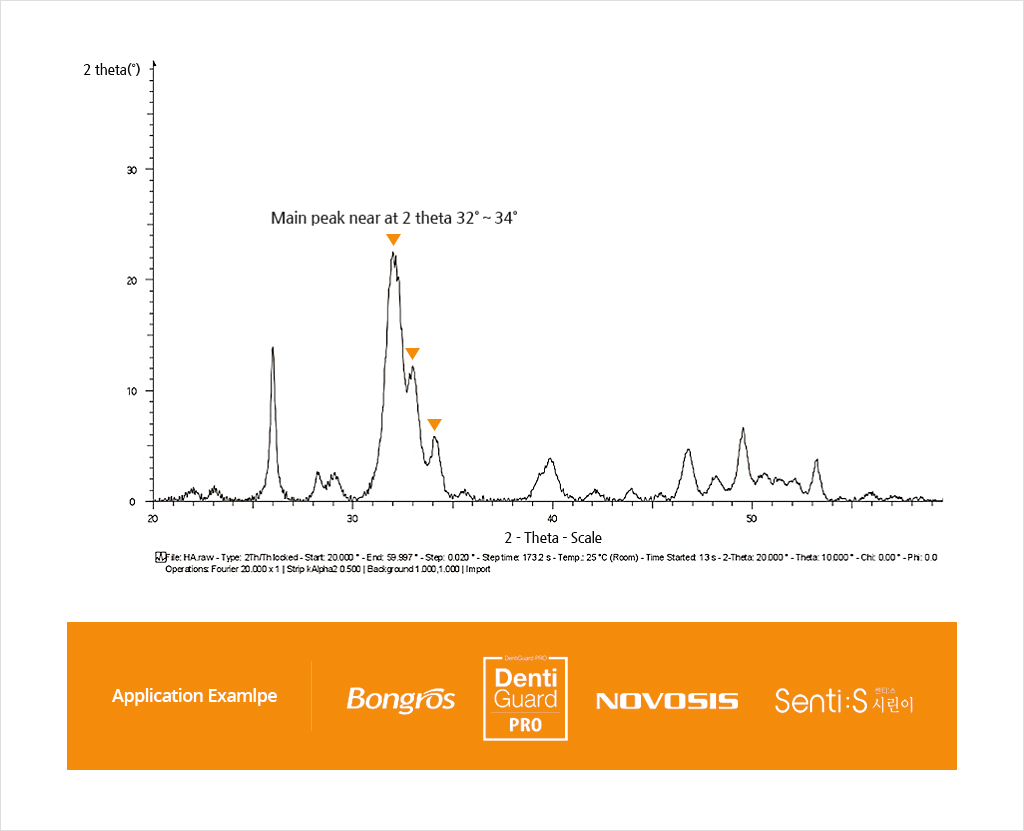
Phase Purity : More than 95% (by XRD)
Crystalline Phase : Low crystallinity hydroxyapatite (by XRD)
Trace Elements Analysis : Arsenic (AS) < 3ppm / Mercury (Hg) < 5ppm,
Cadmium (Cd) < 5ppm / Lead (Pb) < 30ppm, Total heavy metals < 50ppm (by ASTM F1185-03)
XRD TEST ANALYSIS